What are the main problems you encounter when Gram staining?
A few weeks ago, we launched our first survey on Linkedin.
Our question was: "What are the main problems you encounter when Gram staining?"
We received 165 responses, and 39% of our followers told us that your main problem was sample loss during staining.
| Problem | % of responses | |
|---|---|---|
| 1 | Sample lost during staining | 39 |
| 2 | Ring patterns, cell distortions | 29 |
| 3 | Gram-positive organisms appear pink | 21 |
| 4 | Gram-negative organisms appear to be violet | 11 |
We created a carousel about their main problem, the sample lost during staining, but we also developed tips to help you resolve the other problems:
1.Sample loss during staining can be caused by:
Smearing the sample too thick on the slide
Thick samples may not properly adhere to the slide when fixed and can wash off during staining. This problem can be avoided by ensuring a proper thickness when smearing the sample on the slide. A suitable thickness allows one to see and read text on printed paper through the slide at a distance of 10 cm (as illustrated below).
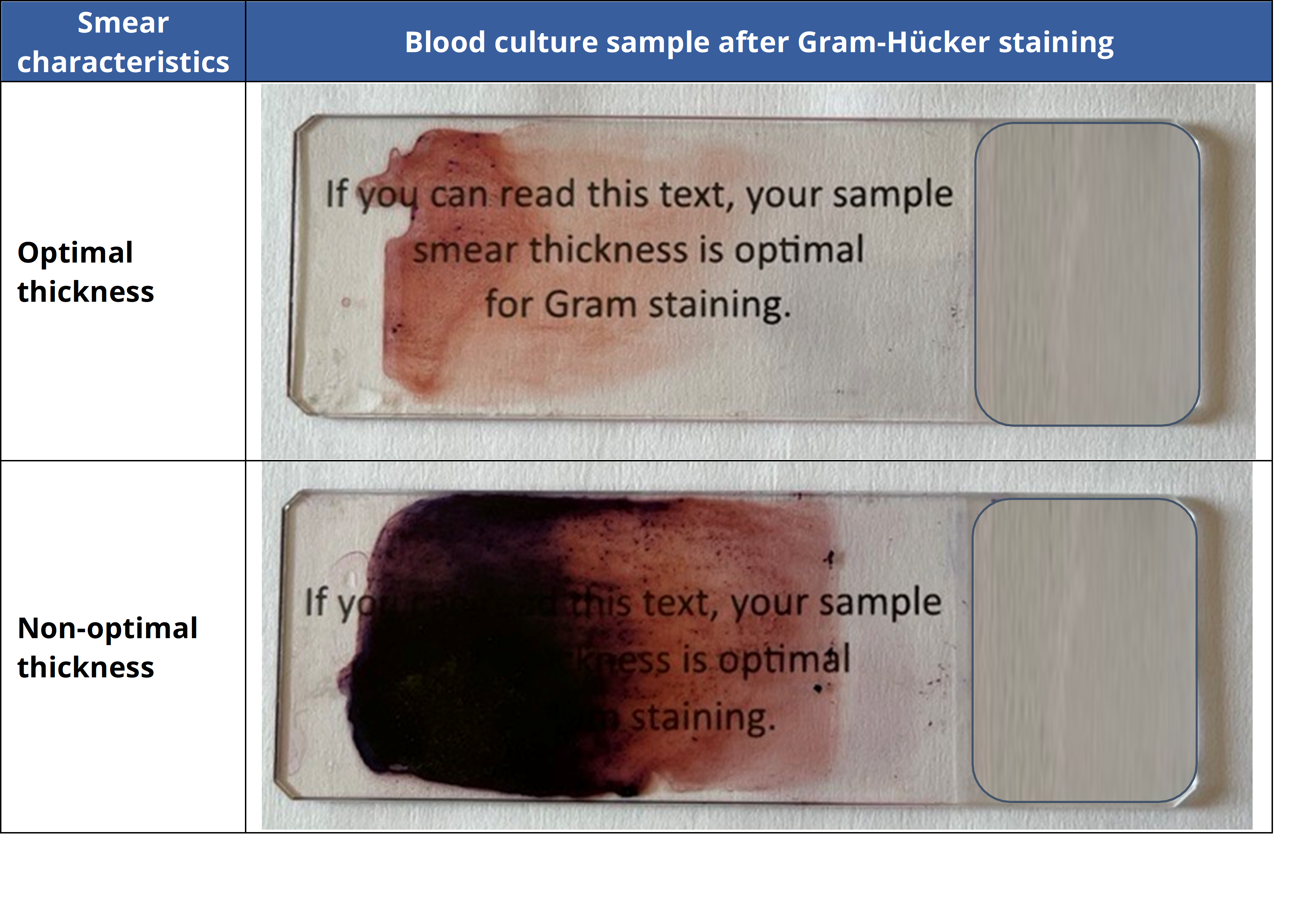
Once the sample has been properly smeared, fixation is highly recommended. Fixation is most often performed using a mild heat source (Bunsen burner or hot plate) or a chemical fixative (methanol, ethanol…).
Otherwise, the two fixation methods can be combined as follows:
Apply a few drops of 95% ethanol to the slide and let sit for a few minutes. Remove the excess ethanol by
- turning the slide over a sink to drain off any excess ethanol or
- allowing the ethanol to evaporate by passing the slide 3 times through the flame of a Bunsen burner or
- placing the slide on a hot plate and allowing the excess alcohol to evaporate. Do not use a temperature above 65°C on the hot plate, as it will damage the cells.
The staining method can also be responsible for sample loss:
if reagents or water are applied directly to the sample or the sample is over-rinsed. We recommend gently applying the reagents and rinsing solution indirectly on the slide during manual staining. Sample loss can be also minimized by decreasing the rinse time on automated stainers.
2.Ring patterns, distortions in cellular morphology, and cellular debris on slides
They are mainly caused by overheating the slide during fixation. The optimal temperature for a hot plate is 65°C. When using a Bunsen burner, ensure the fixed specimen is on top of the slide when passing it 3 times through the flame.
We also recommend ensuring that the sampling method and the conservation of the sample before staining were respected to avoid any sample degradation before analyzing it.
3.Gram-positive bacteria are incorrectly stained and appear pink (Gram-negative).
There are three main causes of this phenomenon:
- 1. The analysis was done in a thick area of the slide.
Modify the reading zone by choosing a thinner area where bacteria are more accurately stained. This will enable a better assessment of the diversity and morphology of bacteria in the sample. The table below illustrates appropriate and inappropriate reading zones in a stool sample.
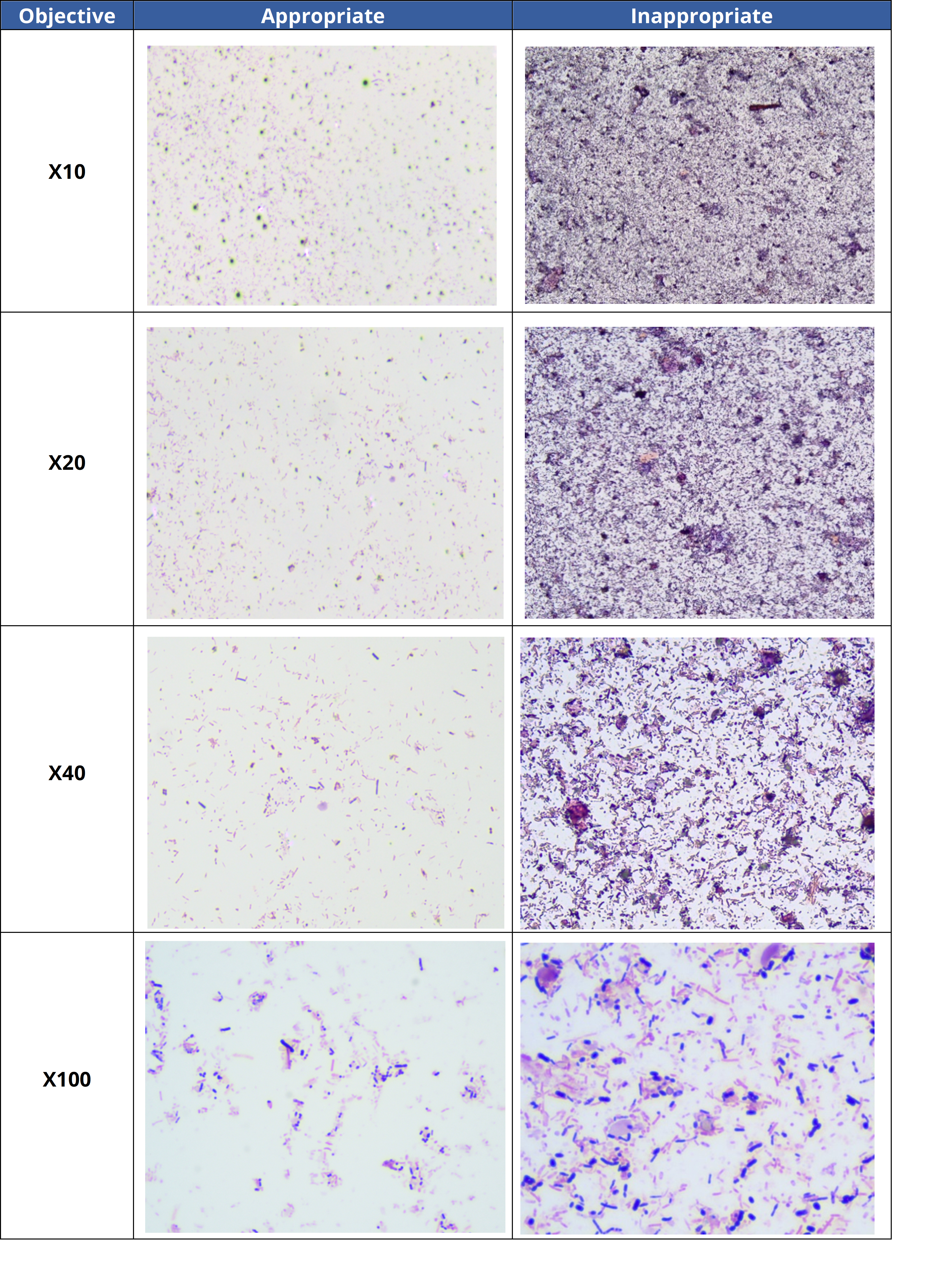
- 2. Technical problem with staining method:
- The sample was over-decolorized. We recommend decreasing bath timing in differentiator-discoloring or rinsing solution.
- Insufficient application time of crystal violet oxalate/carbolic gentian violet or Lugol stain. We recommend increasing bath time in these reagents until the desired level of staining is achieved.
- 3. The sample contains Gram variable bacteria.
Gram-variable bacteria show a mix of pink and purple coloration in the same culture sample when stained. It is important to be aware of the staining characteristics of Gram variable bacteria when analyzing stained slides/specimens. The table below provides common genera with Gram variable species.
| Genus with Gram Variable |
Staining Characteristics |
Causes |
|---|---|---|
|
Actinomyces (+) Arthrobacter (+) Corynebacterium (+) Propionibacterium (+)
|
|
|
|
Bacillus (+) Butyrivibrio (+) Clostridium (+) |
|
The thickness of the peptidoglycan layer becomes thinner as the bacteria get older, causing the leakage of crystal violet/gentian violet stain. |
| Eubacterium (+) (E.lentum, E.cylindroides) |
A mix of pink and purple bacteria are observed in the same sample. |
- |
|
Acinetobacter (-) Moraxella (-) |
There tends to appear purple or a mix of pink and purple bacteria within the same sample. |
The bacteria have a cell structure with a high capacity to resist decolorization and retain crystal violet/gentian violet dye. |
Note: This table contains only a partial list of Gram variable species within a given genus. Gram variable species can be found in other genera as well, for example Lactobacillus, Gardnerella, and Leptotrichia.
4. Gram-negative bacteria are incorrectly stained and appear violet (Gram-positive).
There are four main causes of this phenomenon:
- The analysis was done in a thick area of the slide. We recommend analyzing the slide in a thinner area, as discussed in the previous section.
- The sample was under-decolorized, or the Lugol solution was not adequately drained during staining. This problem can be addressed by increasing the time in the bath differentiator-discoloring or in the Lugol solution.
- Dye deposits/precipitate in bottled reagents can be confused with bacteria. We recommend filtering the solution using a pleated paper filter with a porosity of 5-8 µm.
- The sample contains Gram-variable bacteria. More details are available in the previous section.